Guidelines
The Evidence in Allergy group helps shape optimal care through systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.

American College of Allergy, Asthma and Immunology
Vaccines in Patients Receiving Dupilumab, A Systematic Review and Expert Delphi Consensus Recommendation: A position paper of the American College of Allergy, Asthma and Immunology
American College of Allergy, Asthma and Immunology
Vaccines in Patients Receiving Dupilumab, A Systematic Review and Expert Delphi Consensus Recommendation: A position paper of the American College of Allergy, Asthma and Immunology
June 5, 2024
Background:
Dupilumab is a monoclonal antibody that targets the interleukin (IL)-4 receptor alpha subunit, thus blocking the effects of IL-4 and IL-13, and has shown efficacy in treating various conditions including asthma, atopic dermatitis, eosinophilic esophagitis, and others. Because of its immune modulatory effects, clinical trials that studied dupilumab did not allow patients to receive live vaccines during the clinical trials because of an abundance of caution, and thus package inserts recommend that patients who are being treated with dupilumab should avoid live vaccines. Because dupilumab is now approved for use in patients from 6 months of age for the treatment of atopic dermatitis, this reported contraindication is now posing a clinical dilemma for patients and clinicians.
Objective:
To perform a systematic review of literature on the safety and efficacy of vaccinations in patients who are receiving dupilumab and to provide expert guidance on the use of vaccines in patients who are receiving dupilumab.
Methods:
A systematic review of the literature was performed, and an expert Delphi Panel was assembled.
Results:
The available literature on patients who received vaccinations while using dupilumab overall suggests that live vaccines are safe and that the vaccine efficacy, in general, is not affected by dupilumab. The expert Delphi panel agreed that the use of vaccines in patients receiving dupilumab was likely safe and effective.
Conclusion:
Vaccines (including live vaccines) can be administered to patients receiving dupilumab in a shared decision-making capacity.
Dupilumab is a monoclonal antibody that targets the interleukin (IL)-4 receptor alpha subunit, thus blocking the effects of IL-4 and IL-13, and has shown efficacy in treating various conditions including asthma, atopic dermatitis, eosinophilic esophagitis, and others. Because of its immune modulatory effects, clinical trials that studied dupilumab did not allow patients to receive live vaccines during the clinical trials because of an abundance of caution, and thus package inserts recommend that patients who are being treated with dupilumab should avoid live vaccines. Because dupilumab is now approved for use in patients from 6 months of age for the treatment of atopic dermatitis, this reported contraindication is now posing a clinical dilemma for patients and clinicians.
Objective:
To perform a systematic review of literature on the safety and efficacy of vaccinations in patients who are receiving dupilumab and to provide expert guidance on the use of vaccines in patients who are receiving dupilumab.
Methods:
A systematic review of the literature was performed, and an expert Delphi Panel was assembled.
Results:
The available literature on patients who received vaccinations while using dupilumab overall suggests that live vaccines are safe and that the vaccine efficacy, in general, is not affected by dupilumab. The expert Delphi panel agreed that the use of vaccines in patients receiving dupilumab was likely safe and effective.
Conclusion:
Vaccines (including live vaccines) can be administered to patients receiving dupilumab in a shared decision-making capacity.
Allergy Asthma Immunol. 2024 Jun 5:S1081-1206(24)00337-5. doi: 10.1016/j.anai.2024.05.014.

COMFA - European Cooperation in Science & Technology
Core Outcome Set for IgE-mediated food allergy clinical trials and observational studies of interventions: International Delphi consensus study 'COMFA'
COMFA - European Cooperation in Science & Technology
Core Outcome Set for IgE-mediated food allergy clinical trials and observational studies of interventions: International Delphi consensus study 'COMFA'
March 3, 2024
Background: IgE-mediated food allergy (FA) is a global health concern with substantial individual and societal implications. While diverse intervention strategies have been researched, inconsistencies in reported outcomes limit evaluations of FA treatments. To streamline evaluations and promote consistent reporting, the Core Outcome Measures for Food Allergy (COMFA) initiative aimed to establish a Core Outcome Set (COS) for FA clinical trials and observational studies of interventions.
Methods: The project involved a review of published clinical trials, trial protocols and qualitative literature. Outcomes found as a result of review were categorized and classified, informing a two-round online-modified Delphi process followed by hybrid consensus meeting to finalize the COS.
Results: The literature review, taxonomy mapping and iterative discussions with diverse COMFA group yielded an initial list of 39 outcomes. The iterative online and in-person meetings reduced the list to 13 outcomes for voting in the formal Delphi process. One more outcome was added based on participant suggestions after the first Delphi round. A total of 778 participants from 52 countries participated, with 442 participating in both Delphi rounds. No outcome met a priori criteria for inclusion, and one was excluded as a result of the Delphi. Thirteen outcomes were brought to the hybrid consensus meeting as a result of Delphi and two outcomes, 'allergic symptoms' and 'quality of life' achieved consensus for inclusion as 'core' outcomes.
Conclusion: In addition to the mandatory reporting of adverse events for FA clinical trials or observational studies of interventions, allergic symptoms and quality of life should be measured as core outcomes. Future work by COMFA will define how best to measure these core outcomes.
Methods: The project involved a review of published clinical trials, trial protocols and qualitative literature. Outcomes found as a result of review were categorized and classified, informing a two-round online-modified Delphi process followed by hybrid consensus meeting to finalize the COS.
Results: The literature review, taxonomy mapping and iterative discussions with diverse COMFA group yielded an initial list of 39 outcomes. The iterative online and in-person meetings reduced the list to 13 outcomes for voting in the formal Delphi process. One more outcome was added based on participant suggestions after the first Delphi round. A total of 778 participants from 52 countries participated, with 442 participating in both Delphi rounds. No outcome met a priori criteria for inclusion, and one was excluded as a result of the Delphi. Thirteen outcomes were brought to the hybrid consensus meeting as a result of Delphi and two outcomes, 'allergic symptoms' and 'quality of life' achieved consensus for inclusion as 'core' outcomes.
Conclusion: In addition to the mandatory reporting of adverse events for FA clinical trials or observational studies of interventions, allergic symptoms and quality of life should be measured as core outcomes. Future work by COMFA will define how best to measure these core outcomes.
Allergy. 2024 Mar 3. doi: 10.1111/all.16023. PMID: 38433402.

AAAAI/ACAAI Joint Task Force on Practice Parameters
Atopic dermatitis (eczema) guidelines
AAAAI/ACAAI Joint Task Force on Practice Parameters
Atopic dermatitis (eczema) guidelines
December 18, 2023
Background
Guidance addressing atopic dermatitis (AD) management, last issued in 2012 by the American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force, requires updating as a result of new treatments and improved guideline and evidence synthesis methodology.
Objective
To produce evidence-based guidelines that support patients, clinicians, and other decision-makers in the optimal treatment of AD.
Methods
A multidisciplinary guideline panel consisting of patients and caregivers, AD experts (dermatology and allergy/immunology), primary care practitioners (family medicine, pediatrics, internal medicine), and allied health professionals (psychology, pharmacy, nursing) convened, prioritized equity, diversity, and inclusiveness, and implemented management strategies to minimize influence of conflicts of interest. The Evidence in Allergy Group supported guideline development by performing systematic evidence reviews, facilitating guideline processes, and holding focus groups with patient and family partners. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach informed rating the certainty of evidence and strength of recommendations. Evidence-to-decision frameworks, subjected to public comment, translated evidence to recommendations using trustworthy guideline principles.
Results
The panel agreed on 25 recommendations to gain and maintain control of AD for patients with mild, moderate, and severe AD. The eAppendix provides practical information and implementation considerations in 1-2 page patient-friendly handouts.
Conclusion
These evidence-based recommendations address optimal use of (1) topical treatments (barrier moisturization devices, corticosteroids, calcineurin inhibitors, PDE4 inhibitors [crisaborole], topical JAK inhibitors, occlusive [wet wrap] therapy, adjunctive antimicrobials, application frequency, maintenance therapy), (2) dilute bleach baths, (3) dietary avoidance/elimination, (4) allergen immunotherapy, and (5) systemic treatments (biologics/monoclonal antibodies, small molecule immunosuppressants [cyclosporine, methotrexate, azathioprine, mycophenolate, JAK inhibitors], and systemic corticosteroids) and UV phototherapy (light therapy).
Guidance addressing atopic dermatitis (AD) management, last issued in 2012 by the American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force, requires updating as a result of new treatments and improved guideline and evidence synthesis methodology.
Objective
To produce evidence-based guidelines that support patients, clinicians, and other decision-makers in the optimal treatment of AD.
Methods
A multidisciplinary guideline panel consisting of patients and caregivers, AD experts (dermatology and allergy/immunology), primary care practitioners (family medicine, pediatrics, internal medicine), and allied health professionals (psychology, pharmacy, nursing) convened, prioritized equity, diversity, and inclusiveness, and implemented management strategies to minimize influence of conflicts of interest. The Evidence in Allergy Group supported guideline development by performing systematic evidence reviews, facilitating guideline processes, and holding focus groups with patient and family partners. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach informed rating the certainty of evidence and strength of recommendations. Evidence-to-decision frameworks, subjected to public comment, translated evidence to recommendations using trustworthy guideline principles.
Results
The panel agreed on 25 recommendations to gain and maintain control of AD for patients with mild, moderate, and severe AD. The eAppendix provides practical information and implementation considerations in 1-2 page patient-friendly handouts.
Conclusion
These evidence-based recommendations address optimal use of (1) topical treatments (barrier moisturization devices, corticosteroids, calcineurin inhibitors, PDE4 inhibitors [crisaborole], topical JAK inhibitors, occlusive [wet wrap] therapy, adjunctive antimicrobials, application frequency, maintenance therapy), (2) dilute bleach baths, (3) dietary avoidance/elimination, (4) allergen immunotherapy, and (5) systemic treatments (biologics/monoclonal antibodies, small molecule immunosuppressants [cyclosporine, methotrexate, azathioprine, mycophenolate, JAK inhibitors], and systemic corticosteroids) and UV phototherapy (light therapy).
In Press

AAAAI/ACAAI Joint Task Force on Practice Parameters
Anaphylaxis: A 2023 practice parameter update
AAAAI/ACAAI Joint Task Force on Practice Parameters
Anaphylaxis: A 2023 practice parameter update
December 18, 2023
Abstract
This practice parameter update focuses on 7 areas in which there are new evidence and new recommendations. Diagnostic criteria for anaphylaxis have been revised, and patterns of anaphylaxis are defined. Measurement of serum tryptase is important for diagnosis of anaphylaxis and to identify underlying mast cell disorders. In infants and toddlers, age-specific symptoms may differ from older children and adults, patient age is not correlated with reaction severity, and anaphylaxis is unlikely to be the initial reaction to an allergen on first exposure. Different community settings for anaphylaxis require specific measures for prevention and treatment of anaphylaxis. Optimal prescribing and use of epinephrine autoinjector devices require specific counseling and training of patients and caregivers, including when and how to administer the epinephrine autoinjector and whether and when to call 911. If epinephrine is used promptly, immediate activation of emergency medical services may not be required if the patient experiences a prompt, complete, and durable response. For most medical indications, the risk of stopping or changing beta-blocker or angiotensin-converting enzyme inhibitor medication may exceed the risk of more severe anaphylaxis if the medication is continued, especially in patients with insect sting anaphylaxis. Evaluation for mastocytosis, including a bone marrow biopsy, should be considered for adult patients with severe insect sting anaphylaxis or recurrent idiopathic anaphylaxis. After perioperative anaphylaxis, repeat anesthesia may proceed in the context of shared decision-making and based on the history and results of diagnostic evaluation with skin tests or in vitro tests when available, and supervised challenge when necessary.
This practice parameter update focuses on 7 areas in which there are new evidence and new recommendations. Diagnostic criteria for anaphylaxis have been revised, and patterns of anaphylaxis are defined. Measurement of serum tryptase is important for diagnosis of anaphylaxis and to identify underlying mast cell disorders. In infants and toddlers, age-specific symptoms may differ from older children and adults, patient age is not correlated with reaction severity, and anaphylaxis is unlikely to be the initial reaction to an allergen on first exposure. Different community settings for anaphylaxis require specific measures for prevention and treatment of anaphylaxis. Optimal prescribing and use of epinephrine autoinjector devices require specific counseling and training of patients and caregivers, including when and how to administer the epinephrine autoinjector and whether and when to call 911. If epinephrine is used promptly, immediate activation of emergency medical services may not be required if the patient experiences a prompt, complete, and durable response. For most medical indications, the risk of stopping or changing beta-blocker or angiotensin-converting enzyme inhibitor medication may exceed the risk of more severe anaphylaxis if the medication is continued, especially in patients with insect sting anaphylaxis. Evaluation for mastocytosis, including a bone marrow biopsy, should be considered for adult patients with severe insect sting anaphylaxis or recurrent idiopathic anaphylaxis. After perioperative anaphylaxis, repeat anesthesia may proceed in the context of shared decision-making and based on the history and results of diagnostic evaluation with skin tests or in vitro tests when available, and supervised challenge when necessary.
In Press 2023

EAACI
Diagnosis of IgE mediated food allergy
EAACI
Diagnosis of IgE mediated food allergy
October 10, 2023
Abstract
This European Academy of Allergy and Clinical Immunology guideline provides recommendations for diagnosing IgE-mediated food allergy and was developed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. Food allergy diagnosis starts with an allergy-focused clinical history followed by tests to determine IgE sensitization, such as serum allergen-specific IgE (sIgE) and skin prick test (SPT), and the basophil activation test (BAT), if available. Evidence for IgE sensitization should be sought for any suspected foods. The diagnosis of allergy to some foods, such as peanut and cashew nut, is well supported by SPT and serum sIgE, whereas there are less data and the performance of these tests is poorer for other foods, such as wheat and soya. The measurement of sIgE to allergen components such as Ara h 2 from peanut, Cor a 14 from hazelnut and Ana o 3 from cashew can be useful to further support the diagnosis, especially in pollen-sensitized individuals. BAT to peanut and sesame can be used additionally. The reference standard for food allergy diagnosis is the oral food challenge (OFC). OFC should be performed in equivocal cases. For practical reasons, open challenges are suitable in most cases. Reassessment of food allergic children with allergy tests and/or OFCs periodically over time will enable reintroduction of food into the diet in the case of spontaneous acquisition of oral tolerance.
This European Academy of Allergy and Clinical Immunology guideline provides recommendations for diagnosing IgE-mediated food allergy and was developed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. Food allergy diagnosis starts with an allergy-focused clinical history followed by tests to determine IgE sensitization, such as serum allergen-specific IgE (sIgE) and skin prick test (SPT), and the basophil activation test (BAT), if available. Evidence for IgE sensitization should be sought for any suspected foods. The diagnosis of allergy to some foods, such as peanut and cashew nut, is well supported by SPT and serum sIgE, whereas there are less data and the performance of these tests is poorer for other foods, such as wheat and soya. The measurement of sIgE to allergen components such as Ara h 2 from peanut, Cor a 14 from hazelnut and Ana o 3 from cashew can be useful to further support the diagnosis, especially in pollen-sensitized individuals. BAT to peanut and sesame can be used additionally. The reference standard for food allergy diagnosis is the oral food challenge (OFC). OFC should be performed in equivocal cases. For practical reasons, open challenges are suitable in most cases. Reassessment of food allergic children with allergy tests and/or OFCs periodically over time will enable reintroduction of food into the diet in the case of spontaneous acquisition of oral tolerance.
Allergy. 2023 Dec;78(12):3057-3076.

International Guidelines
Updated Guidance Regarding The Risk ofAllergic Reactions to COVID-19 Vaccines and Recommended Evaluation and Management: A GRADE Assessment, and International Consensus Approach
International Guidelines
Updated Guidance Regarding The Risk ofAllergic Reactions to COVID-19 Vaccines and Recommended Evaluation and Management: A GRADE Assessment, and International Consensus Approach
June 7, 2023
This guidance updates 2021 GRADE (Grading of Recommendations Assessment, Development and Evaluation) recommendations regarding immediate allergic reactions following coronavirus disease 2019 (COVID-19) vaccines and addresses revaccinating individuals with first-dose allergic reactions and allergy testing to determine revaccination outcomes. Recent meta-analyses assessed the incidence of severe allergic reactions to initial COVID-19 vaccination, risk of mRNA-COVID-19 revaccination after an initial reaction, and diagnostic accuracy of COVID-19 vaccine and vaccine excipient testing in predicting reactions. GRADE methods informed rating the certainty of evidence and strength of recommendations. A modified Delphi panel consisting of experts in allergy, anaphylaxis, vaccinology, infectious diseases, emergency medicine, and primary care from Australia, Canada, Europe, Japan, South Africa, the United Kingdom, and the United States formed the recommendations. We recommend vaccination for persons without COVID-19 vaccine excipient allergy and revaccination after a prior immediate allergic reaction. We suggest against >15-minute postvaccination observation. We recommend against mRNA vaccine or excipient skin testing to predict outcomes. We suggest revaccination of persons with an immediate allergic reaction to the mRNA vaccine or excipients be performed by a person with vaccine allergy expertise in a properly equipped setting. We suggest against premedication, split-dosing, or special precautions because of a comorbid allergic history.
J Allergy Clin Immunol . 2023 Aug;152(2):309-325. doi: 10.1016/j.jaci.2023.05.019. Epub 2023 Jun 7.

AAAAI/ACAAI Joint Task Force on Practice Parameters
The Joint Task Force on Practice Parameters GRADE Guidelines for the Medical Management of Chronic Rhinosinusitis with Nasal Polyposis
AAAAI/ACAAI Joint Task Force on Practice Parameters
The Joint Task Force on Practice Parameters GRADE Guidelines for the Medical Management of Chronic Rhinosinusitis with Nasal Polyposis
November 9, 2022
These evidence-based guidelines support patients, clinicians, and other stakeholders in decisions about the use of intranasal corticosteroids (INCS), biologics, and aspirin therapy after desensitization (ATAD) for the management of chronic rhinosinusitis with nasal polyposis (CRSwNP). It is important to note that the current evidence on surgery for CRSwNP was not assessed for this guideline, nor were management options other than INCS, biologics, and ATAD. The Allergy-Immunology Joint Task Force on Practice Parameters formed a multidisciplinary guideline panel balanced to include the views of multiple stakeholders and to minimize potential biases. Systematic reviews for each management option informed the guideline. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to inform and develop recommendations. The guideline panel reached consensus on the following statements: (1) In people with CRSwNP, the guideline panel suggests INCS rather than no INCS (conditional recommendation, low certainty of evidence) (2) In people with CRSwNP, the guideline panel suggests biologics rather than no biologics (conditional recommendation, moderate certainty of evidence) (3) In people with aspirin (nonsteroidal anti-inflammatory drug) exacerbated respiratory disease, the guideline panel suggests ATAD rather than no ATAD (conditional recommendation, moderate certainty of evidence). The conditions for each recommendation are discussed in the guideline.
JACI 2022. Epub online Nov 2022

AAAAI/ACAAI Joint Task Force on Practice Parameters
Drug Allergy: A 2022 Practice Parameter Update
AAAAI/ACAAI Joint Task Force on Practice Parameters
Drug Allergy: A 2022 Practice Parameter Update
September 16, 2022
This practice parameter provides an updated approach to the diagnosis and management of various drug reactions. Evidence has evolved since the previous drug allergy practice parameter and currently supports the ability to risk stratify most patients based upon reaction phenotype. Evaluation of suspected drug allergy focuses on preferential utilization of drug challenges as opposed to skin testing in many circumstances. Clarification of drug allergy history is a valuable resource that allergist-immunologists provide to patients with shared decision making regarding testing and management options central to each evaluation. These parameters will help clinicians better understand how and when to utilize drug challenges, including consideration for 1-, 2-, or multi-step challenges. A proactive approach to delabeling penicillin allergy as well as use of safe antibiotic alternatives for patients with proven penicillin allergy is emphasized. Approaches to diagnosis and management of non-penicillin drug reactions are discussed in updated sections on cephalosporins, sulfonamides, fluroquinolones, macrolides, aspirin, chemotherapeutic agents, and biologics.

World Allergy Organization
Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA)
World Allergy Organization
Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA)
December 10, 2021
Since the World Allergy Organization (WAO) Diagnosis and Rationale against Cow's Milk Allergy (DRACMA) Guidelines were published 10 years ago, new evidence has accumulated about the diagnosis, therapy, and specific immunotherapy for cow's milk allergy (CMA). For this reason, WAO has felt the need to update the guidelines.
We introduce here this update. The new DRACMA guidelines aim to comprehensively address the guidance on diagnosis and therapy of both IgE non-IgE-mediated forms of cow's milk allergy in children and adults. They will be divided into 18 chapters, each of which will be dedicated to an aspect. The focus will be on the meta-analyzes and recommendations that will be expressed for the 3 most relevant clinical aspects: (a) the diagnostic identification of the condition; (b) the choice of the replacement formula in case of CMA in infancy when the mother is not able to breastfeed, and (c) the use of specific immunotherapy for cow's milk protein allergy.
We introduce here this update. The new DRACMA guidelines aim to comprehensively address the guidance on diagnosis and therapy of both IgE non-IgE-mediated forms of cow's milk allergy in children and adults. They will be divided into 18 chapters, each of which will be dedicated to an aspect. The focus will be on the meta-analyzes and recommendations that will be expressed for the 3 most relevant clinical aspects: (a) the diagnostic identification of the condition; (b) the choice of the replacement formula in case of CMA in infancy when the mother is not able to breastfeed, and (c) the use of specific immunotherapy for cow's milk protein allergy.

International Guidelines
The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and International Consensus Approach
International Guidelines
The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and International Consensus Approach
October 1, 2021
Concerns for anaphylaxis may hamper severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunization efforts. We convened a multidisciplinary group of international experts in anaphylaxis composed of allergy, infectious disease, emergency medicine, and front-line clinicians to systematically develop recommendations regarding SARS-CoV-2 vaccine immediate allergic reactions. Medline, EMBASE, Web of Science, the World Health Organizstion (WHO) global coronavirus database, and the gray literature (inception, March 19, 2021) were systematically searched. Paired reviewers independently selected studies addressing anaphylaxis after SARS-CoV-2 vaccination, polyethylene glycol (PEG) and polysorbate allergy, and accuracy of allergy testing for SARS-CoV-2 vaccine allergy. Random effects models synthesized the data to inform recommendations based on the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach, agreed upon using a modified Delphi panel. The incidence of SARS-CoV-2 vaccine anaphylaxis is 7.91 cases per million (n = 41,000,000 vaccinations; 95% confidence interval [95% CI] 4.02-15.59; 26 studies, moderate certainty), the incidence of 0.15 cases per million patient-years (95% CI 0.11-0.2), and the sensitivity for PEG skin testing is poor, although specificity is high (15 studies, very low certainty). We recommend vaccination over either no vaccination or performing SARS-CoV-2 vaccine/excipient screening allergy testing for individuals without history of a severe allergic reaction to the SARS-CoV-2 vaccine/excipient, and a shared decision-making paradigm in consultation with an allergy specialist for individuals with a history of a severe allergic reaction to the SARS-CoV-2 vaccine/excipient. We recommend further research to clarify SARS-CoV-2 vaccine/vaccine excipient testing utility in individuals potentially allergic to SARS-CoV2 vaccines or their excipients.
J Allergy Clin Immunol Pract. 2021 Oct; 9(10): 3546–3567.

GRADE
Addressing imprecision in network meta-analysis
GRADE
Addressing imprecision in network meta-analysis
July 19, 2021
Objective
This article describes GRADE guidance for assessing imprecision when rating the certainty of the evidence from network meta-analysis.
Study Design and Setting
A project group within the GRADE working group conducted iterative discussions, computer simulations, and presentations at GRADE working group meetings to produce and obtain approval for this guidance.
Results
When addressing imprecision of a network estimate, reviewers should consider the 95% confidence or credible interval, and the optimal information size. If the 95% confidence or credible interval crosses a pre-specified threshold, reviewers should rate down the certainty of the evidence. If the 95% confidence interval does not cross any pre-specfied threshold, reviewers should consider the optimal information size. Because addressing the optimal information size may be challenging, reviewers can use the effect size to decide if any calculations are necessary. When the size of the effect is modest or the optimal information size is met, reviewers should not rate down for imprecision.
Conclusion
Reviewers should use this guidance when to appropriately address imprecision in the context of the assessment of certainty of evidence from network meta-analysis.
This article describes GRADE guidance for assessing imprecision when rating the certainty of the evidence from network meta-analysis.
Study Design and Setting
A project group within the GRADE working group conducted iterative discussions, computer simulations, and presentations at GRADE working group meetings to produce and obtain approval for this guidance.
Results
When addressing imprecision of a network estimate, reviewers should consider the 95% confidence or credible interval, and the optimal information size. If the 95% confidence or credible interval crosses a pre-specified threshold, reviewers should rate down the certainty of the evidence. If the 95% confidence interval does not cross any pre-specfied threshold, reviewers should consider the optimal information size. Because addressing the optimal information size may be challenging, reviewers can use the effect size to decide if any calculations are necessary. When the size of the effect is modest or the optimal information size is met, reviewers should not rate down for imprecision.
Conclusion
Reviewers should use this guidance when to appropriately address imprecision in the context of the assessment of certainty of evidence from network meta-analysis.
J Clin Epidemiol . 2021 Nov;139:49-56. doi: 10.1016/j.jclinepi.2021.07.011. Epub 2021 Jul 19.

McMaster University
Canada's Global Nexus for Pandemics and Biological Threats
McMaster University
Canada's Global Nexus for Pandemics and Biological Threats
May 25, 2021
Canada’s Global Nexus for Pandemics and Biological Threats builds on McMaster’s established position as a world leader in infectious disease research, evidence-based medicine, and advanced manufacturing. Hosted at the university, it is a hub for international networks of experts and partners who can act fast and act together in the face of serious and emerging threats to global well-being.

International Guidelines
Prevention and Management of Adverse Reactions to Food in Schools McMaster International Guidelines
International Guidelines
Prevention and Management of Adverse Reactions to Food in Schools McMaster International Guidelines
May 1, 2021
Food allergy management in child care centers and schools is a controversial topic, for which evidence-based guidance is needed. Following the Grading of Recommendations Assessment, Development, and Evaluation approach, we conducted systematic literature reviews of the anticipated health effects of selected interventions for managing food allergy in child care centers and schools; we compiled data about the costs, feasibility, acceptability, and effects on health equity of the selected interventions; and we developed the following conditional recommendations: we suggest that child care centers and schools implement allergy training and action plans; we suggest that they use epinephrine (adrenaline) to treat suspected anaphylaxis; we suggest that they stock unassigned epinephrine autoinjectors, instead of requiring students to supply their own personal autoinjectors to be stored on site for designated at-school use; and we suggest that they do not implement site-wide food prohibitions (eg, “nut-free” schools) or allergen-restricted zones (eg, “milk-free” tables), except in the special circumstances identified in this document. The recommendations are labeled “conditional” due to the low quality of available evidence. More research is needed to determine with greater certainty which interventions are likely to be the most beneficial. Policymakers might need to adapt the recommendations to fit local circumstances.

EAACI
Biologicals Guidelines—Recommendations for severe asthma
EAACI
Biologicals Guidelines—Recommendations for severe asthma
January 1, 2021
Severe asthma imposes a significant burden on patients, families and healthcare systems. Management is difficult, due to disease heterogeneity, co-morbidities, complexity in care pathways and differences between national or regional healthcare systems. Better understanding of the mechanisms has enabled a stratified approach to the management of severe asthma, supporting the use of targeted treatments with biologicals. However, there are still many issues that require further clarification. These include selection of a certain biological (as they all target overlapping disease phenotypes), the definition of response, strategies to enhance the responder rate, the duration of treatment and its regimen (in the clinic or home-based) and its cost-effectiveness. The EAACI Guidelines on the use of biologicals in severe asthma follow the GRADE approach in formulating recommendations for each biological and each outcome. In addition, a management algorithm for the use of biologicals in the clinic is proposed, together with future approaches and research priorities.
Allergy . 2021 Jan;76(1):14-44.

AAAAI/ACAAI
Joint Task Force on Practice Parameters (JTFPP) member
AAAAI/ACAAI
Joint Task Force on Practice Parameters (JTFPP) member
January 1, 2021
The Joint Task Force on Practice Parameters (JTFPP) was formed in 1989 to develop practice parameters for diagnosis and management of allergic and immunologic diseases. Members of the JTFPP represent the following organizations:
American Academy of Allergy, Asthma &Immunology (AAAAI)
American College of Allergy, Asthma &Immunology (ACAAI)
American Academy of Allergy, Asthma &Immunology (AAAAI)
American College of Allergy, Asthma &Immunology (ACAAI)

COMFA
Core outcome sets for food allergy
COMFA
Core outcome sets for food allergy
November 1, 2020
Core Outcome sets ensure that trial outcomes are relevant to patients, clinicians, healthcare providers and regulators; and they allow trial outcomes to be combined in meta-analysis, so that new findings are capitalized on as soon as possible. The Core Outcome Measures for Food Allergy (COMFA) project is a multidisciplinary network involving all relevant stakeholders aiming to advance food allergy research and innovation by (a) defining the scope and applicability of food allergy Core Outcome sets; (b) developing Core Outcome sets and measurement tools for food allergy; (c) reaching a consensus on terminology and definitions of measurement properties for food allergy Core Outcomes.

AAAAI/ACAAI Joint Task Force on Practice Parameters
Diagnosis of peanut allergy
AAAAI/ACAAI Joint Task Force on Practice Parameters
Diagnosis of peanut allergy
August 15, 2020
Given the burden of disease and the consequences of a diagnosis of peanut allergy, it is important that peanut allergy be accurately diagnosed so that an appropriate treatment plan can be developed. However, a test that indicates there is peanut sensitization present (eg, a “positive” test) is not always associated with clinical reactivity. This practice parameter addresses the diagnosis of IgE-mediated peanut allergy, both in children and adults, as pertaining to 3 fundamental questions, and based on the systematic reviews and meta-analyses, makes recommendations for the clinician who is evaluating a patient for peanut allergy. These questions relate to when diagnostic tests should be completed, which diagnostic tests to utilize, and the utility (or lack thereof) of diagnostic testing to predict the severity of a future allergic reaction to peanut.
J Allergy Clin Immunol . 2020 Dec;146(6):1302-1334. doi: 10.1016/j.jaci.2020.07.031. Epub 2020 Aug 15.



EAACI
Research Outreach Committee
EAACI
Research Outreach Committee
January 1, 2020
Allergy, asthma and clinical immunology research has many challenges, including complex European regulations, funding opportunities, data gathering and overall quality control. To address these unmet needs, the newly formed EAACI Research and Outreach Committee (EAACI ROC) will develop an innovative research and knowledge exchange network, which will ensure the position of the EAACI as the global leader in promoting and supporting Research and Development.
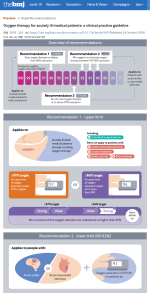
BMJ rapid recommendations
Oxygen therapy in acute ill adults
BMJ rapid recommendations
Oxygen therapy in acute ill adults
November 1, 2018
What is the best way to use oxygen therapy for patients with an acute medical illness? A systematic review published in the Lancet in April 2018 found that supplemental oxygen in inpatients with normal oxygen saturation increases mortality. Its authors concluded that oxygen should be administered conservatively, but they did not make specific recommendations on how to do it. An international expert panel used that review to inform this guideline. It aims to promptly and transparently translate potentially practice-changing evidence to usable recommendations for clinicians and patients. The panel used the GRADE framework and following standards for trustworthy guidelines.
BMJ 2018;363:k4169

GRADE
GRADE working group
GRADE
GRADE working group
January 1, 2018
The Grading of Recommendations Assessment, Development and Evaluation (short GRADE) working group began in the year 2000 as an informal collaboration of people with an interest in addressing the shortcomings of grading systems in health care. The working group has developed a common, sensible and transparent approach to grading quality (or certainty) of evidence and strength of recommendations. Many international organizations have provided input into the development of the GRADE approach which is now considered the standard in guideline development.
BMJ 2008;336:924

AAAAI/ACAAI Joint Task Force on Practice Parameters
Atopic dermatitis - Co-Chair of guidelines
AAAAI/ACAAI Joint Task Force on Practice Parameters
Atopic dermatitis - Co-Chair of guidelines
May 9, 2014
This parameter was developed by the Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma & Immunology (AAAAI); the American College of Allergy, Asthma & Immunology (ACAAI); and the Joint Council of Allergy, Asthma and Immunology. The AAAAI and the ACAAI have jointly accepted responsibility for establishing "Atopic dermatitis: a practice parameter update 2012." This is a complete and comprehensive document at the current time. The medical environment is a changing environment, and not all recommendations will be appropriate for all patients. Because this document incorporated the efforts of many participants, no single individual, including those who served on the Joint Task Force, is authorized to provide an official AAAAI or ACAAI interpretation of these practice parameters. Any request for information about or an interpretation of these practice parameters by the AAAAI or ACAAI should be directed to the Executive Offices of the AAAAI, the ACAAI, and the Joint Council of Allergy, Asthma & Immunology. These parameters are not designed for use by pharmaceutical companies in drug promotion. Published practice parameters of the Joint Task Force on Practice Parameters for Allergy & Immunology are available online at http://www.jcaai.org.
J Allergy Clin Immunol . 2013 Feb;131(2):295-9.e1-27. doi: 10.1016/j.jaci.2012.12.672.

ARIA
Allergic Rhinitis and its Impact on Asthma
ARIA
Allergic Rhinitis and its Impact on Asthma
November 1, 2012
Allergic rhinitis (AR) and asthma represent global health problems for all age groups. Asthma and rhinitis frequently coexist in the same subjects. Allergic Rhinitis and its Impact on Asthma (ARIA) was initiated during a World Health Organization workshop in 1999 (published in 2001). ARIA has reclassified AR as mild/moderate-severe and intermittent/persistent. This classification closely reflects patients' needs and underlines the close relationship between rhinitis and asthma. Patients, clinicians, and other health care professionals are confronted with various treatment choices for the management of AR. This contributes to considerable variation in clinical practice, and worldwide, patients, clinicians, and other health care professionals are faced with uncertainty about the relative merits and downsides of the various treatment options. In its 2010 Revision, ARIA developed clinical practice guidelines for the management of AR and asthma comorbidities based on the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system. ARIA is disseminated and implemented in more than 50 countries of the world. Ten years after the publication of the ARIA World Health Organization workshop report, it is important to make a summary of its achievements and identify the still unmet clinical, research, and implementation needs to strengthen the 2011 European Union Priority on allergy and asthma in children.



